Chemistry & Physics
Click on the subject you want to study
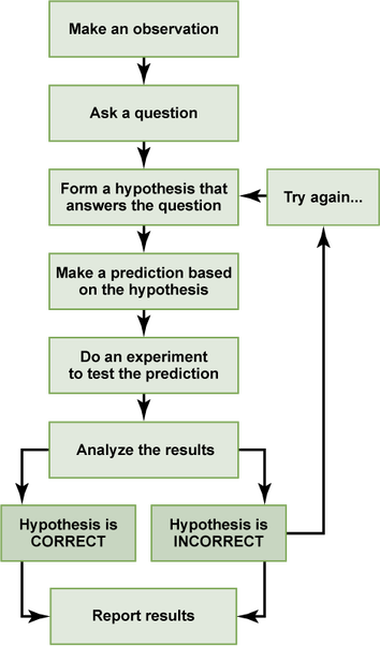
The Scientific MethodThe scientific method is a process that is used to find answers to questions about the world around us. There are several versions of the scientific method. Some have more steps, while others have only a few. All versions of the scientific method include a question to be answered, a hypothesis, and an organized method for conducting and analyzing an experiment.
Read more about the different steps of the scientific method here, and then try and match the different aspects of the scientific method to their definitions in the activity below. |
Properties & States of Matter
|
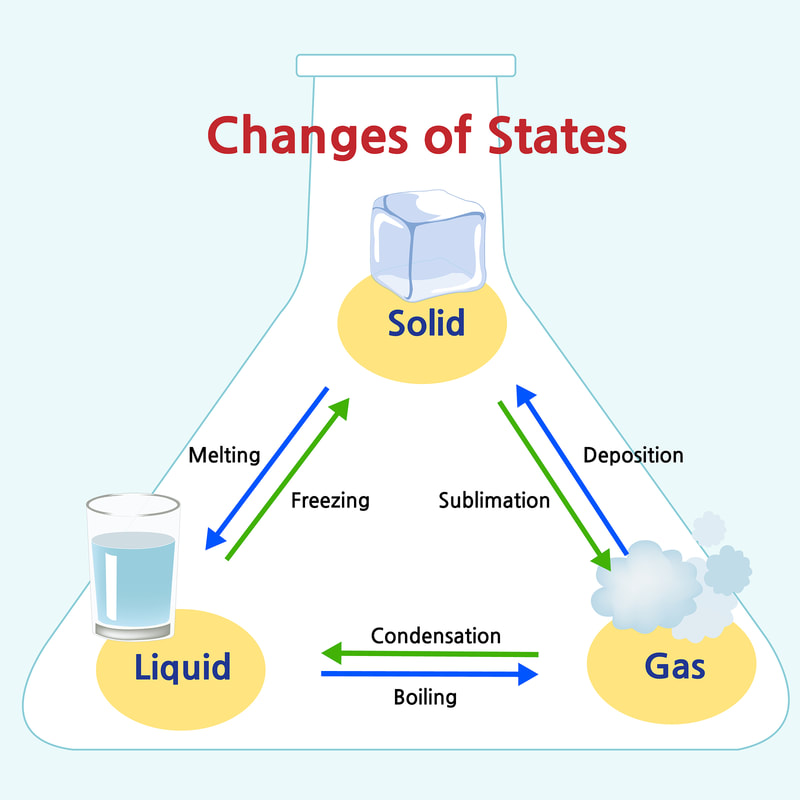
Matter is another word for the stuff things are made of. Everything around us is made of matter, from the air we breath to the water we drink, and even our own bodies. The Earth is made of matter, as well as all the starts, planets, and moons in the universe. All matter is made up of tiny particles called atoms. Matter takes on different forms depending on how the atoms are arranged, and we call these forms the "states of matter." The three most common states of matter are solids, liquids, and gases.
Look at the list of items in the "What is Matter?" activity. Which of the items are matter, and which are not matter? |
|
|
Place characteristics of the states of matter into the correct place on the diagram.
|
Solids, such as wood and stone, have a fixed shape that is difficult to change. This is because the particles inside them are linked to each other and fixed in place.
Liquids, such as water, can flow freely, changing their shape. This is because the particles that make up a liquid are not fixed in place. They stay linked together, but can move around each other. Gases, such as air, are the lightest state of matter. They are easy to push through, and may not be visible to human eyes. The particles in a gas are not connected to each other, so they can float around freely. |
Matter can change its state depending on the temperature. For example, water is a liquid at room temperature, but becomes a solid (called ice) if it is cooled down. The same water turns into a gas (called water vapor) if it is heated up. Changes of matter only happen when the substance reaches a particular temperature.
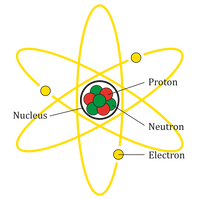
Atoms & MoleculesAll matter is made up of atoms, which are so small that they can be seen only by using a very strong microscope. All atoms have the same basic parts including protons, neutrons, and electrons. Protons and neutrons form the nucleus in the center of the atom, while electrons orbit quickly around the nucleus. The majority of the mass of an atom is in its nucleus. Atoms of different elements differ in the number of protons, electrons and neutrons they have, and this affects their mass.
|
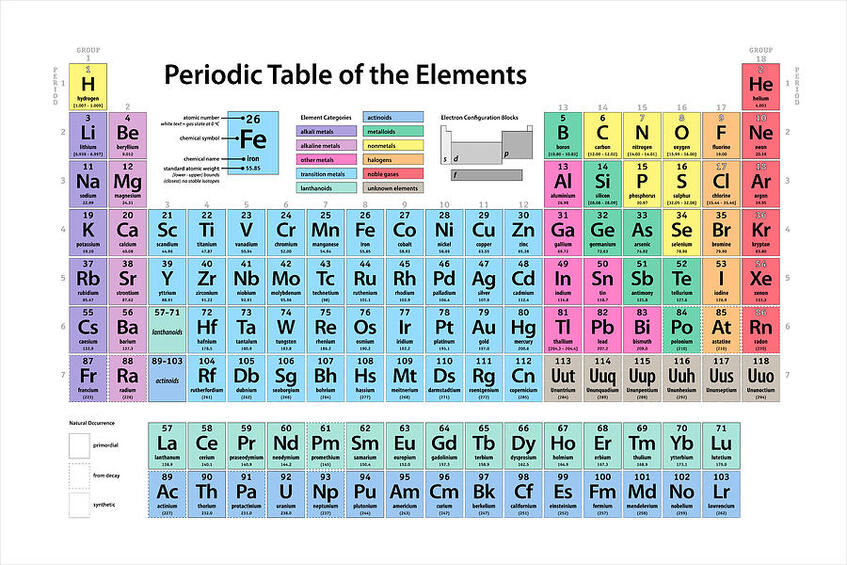
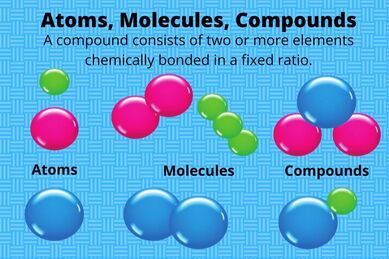
Atoms combine to form elements. One atom of an element is the smallest unit of an element that retains the properties of that element. Each element is given a name, such as hydrogen, gold, silver, helium, and iron, along with a symbol. The element’s name and symbol identify it. Most, but not all, of these symbols are taken from the first or first and second letters of the name of the element, such as H for hydrogen and Ca for calcium. Each atom of a particular element is alike in having the same number of protons in its nucleus. This number is the element’s atomic number. For example, any atom that contains exactly 47 protons in its nucleus is an atom of silver. Any atom that contains only one proton is an atom of hydrogen, the lightest element.
You can explore the elements on an interactive website by clicking on the Periodic Table below:
You can explore the elements on an interactive website by clicking on the Periodic Table below:
You can practice learning the periodic table using the following link. You will need to sign up and get an account, but the account is free.
quizlet.com/4174/the-periodic-table-of-the-elements-flash-cards/
quizlet.com/4174/the-periodic-table-of-the-elements-flash-cards/
|
Two or more atoms that are held together form a molecule. Two atoms of the same element can join together to form a molecule of that element. Molecules are always in motion in each of the three phases of matter, and the speed of the molecules determines the matter’s phase. For example, if the molecules are spread far apart, are moving very fast, and bouncing off one another, the matter of which they are a part is a gas. If they are packed close together and barely moving, they are a part of a solid. If the molecules are moving freely around each other, they are a part of a liquid, which can be poured.
|
|
|
Download the worksheet to review what you've learned about atoms and molecules: |
| ||
Compounds & Counting AtomsCompounds are formed when the atoms of two or more elements join together. Water is an example of a compound, since it is make up of hydrogen and oxygen atoms.
A chemical formula tells us the number of atoms of each element in a compound. It contains the symbols of the atoms of the elements present in the compound as well as how many there are for each element in the form of subscripts. Watch the video to learn about how you can count the atoms in a chemical formula, and download the worksheets to practice counting atoms on your own:
|
| ||||||
Balancing Chemical Equations
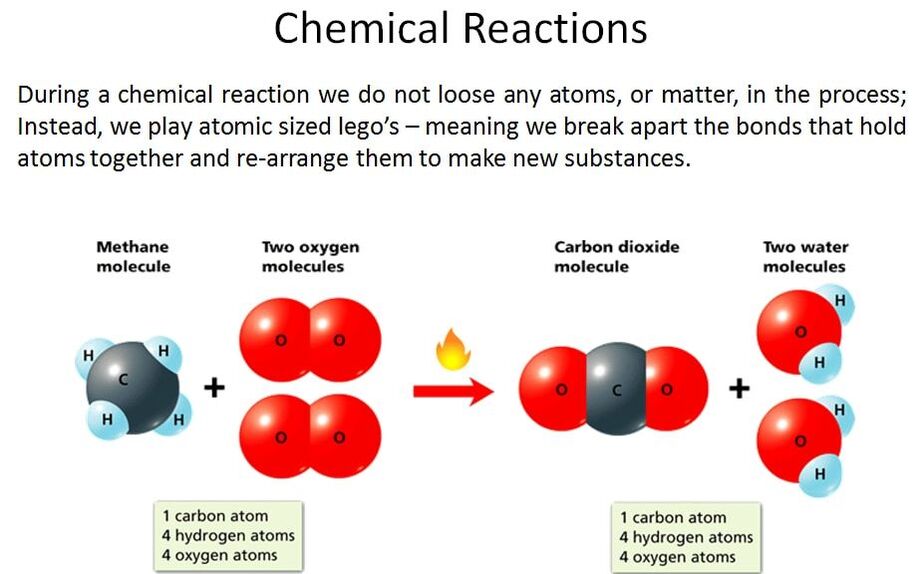
A chemical equation is written representation, using numbers and symbols, of the process that occurs during a chemical reaction. Chemical equations may be either unbalanced or balanced. A balanced chemical equation has the same number and types of atoms on both sides of the arrow. An unbalanced equation lists the reactants and products, but not the ratio between them. Because matter cannot be created or destroyed, chemical equations must be balanced,
|
Watch the video to learn how to balance chemical equations, and download the worksheet to practice balancing them:
| |||
Solubility
|
|
Solubility is the ability of a solid, liquid, or gaseous chemical substance (referred to as the solute) to dissolve in solvent (usually a liquid) and form a solution. The solubility of a substance fundamentally depends on the solvent used, as well as temperature and pressure. Solubility increases with temperature for most substances; for example, more sugar will dissolve in hot water than in cold water. The solubility of a substance in a particular solvent is measured by the concentration of the saturated solution. A solution is considered saturated when adding additional solute no longer increases the concentration of the solution.
Here's a fun solubility experiment that you can try at home!
|
Electricity
Everything in the universe is made of tiny objects called atoms. Each atom has even tinier particles called protons and electrons. These tiny particles swirl around each other continuously. An electron has what is called a negative charge. A proton has a positive charge. Positive and negative charges try to pull each other together. However, two positive charges, or two negative charges, will push each other away. Electricity results when electrons are pushed and pulled from atom to atom. Some atoms lose their electrons more easily than others, so those elements are better to use when moving electricity.
|
Elements like metals lose their electrons very easily. When that happens, the charge of the object changes and the movement of the charged particles creates an electric current. Things that move electrons easily are called conductors. Things that don't move electrons easily are called insulators.
Watch this video to learn more about how electricity works, and then test your knowledge of conductors and insulators by doing the online activity: |
Here is an additional reading for you to look at, and a fun electricity experiment you can try at home!
| ||
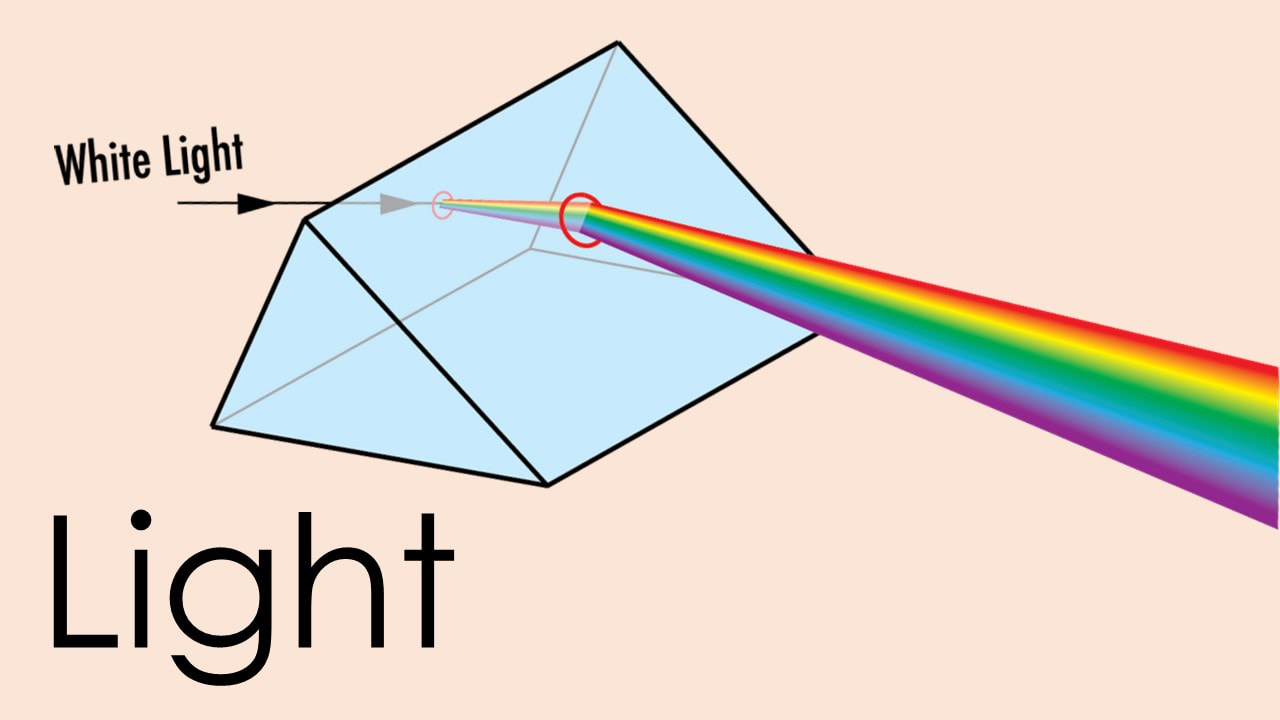
Light
|
Light is electromagnetic radiation that can be detected by the human eye. Electromagnetic radiation occurs over an extremely wide range of wavelengths, from gamma rays to radio waves. Within that broad spectrum, the wavelengths visible to humans occupy a very narrow band, and that is what we call light. Light is made up of packets of energy called photons that move from the source of light in a stream at a very fast speed.
Watch the video to learn more about how we can measure the speed of light and see how it travels. Download the files to read about how rainbows are formed by light, and answer the comprehension questions. Download the GED questions to read more about the electromagnetic spectrum and answer practice questions:
|
| ||||||
Motions & Forces
|
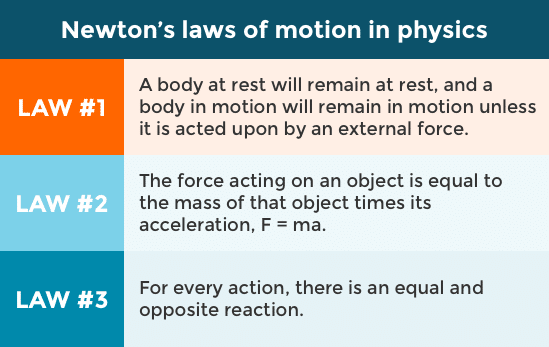
The three laws of motion were first written down by Isaac Newton in the 17th century, and they describe the rules of motion of material objects.
|
Watch this video for a more in depth description of the three laws of motion, or read more about them here.
Test your knowledge of important force and motion vocabulary in this online quiz.
Download this worksheet to practice applying the laws of motion to real-life examples.
| ||||
Chemistry Review
Take this GED practice test to review all you've learned this quarter:
| chemistry_review.pdf |